SUCROSE Rooted in quality:
rising from beet farm to GMP excellence
Experience sucrose that sets new benchmarks and fully complies with the rigorous standards of EP, BP, NF, JP, and ChP. At CG Pharma & Biotech, we are proud to offer a sucrose supply concept thoughtfully developed to not only meet, but exceed the strict requirements of these renowned compendia.

Proven compendial compliance:
- With EP, BP, NF, JP and ChP certifications you can trust that your products are built on a foundation of uncompromising quality.
- Every batch is rigorously analysed, leaving no room for uncertainty.
- Low endotoxin levels: our multi-compendial sucrose boasts low endotoxin levels, offering an added layer of purity and safety to your products.
- Filling of sucrose into a wide range of packaging (e.g. 1 kg - 25 kg) under cleanroom conditions.
- Ensuring year-round availability through stock management, dedicated ware-housing and robust production capacity.
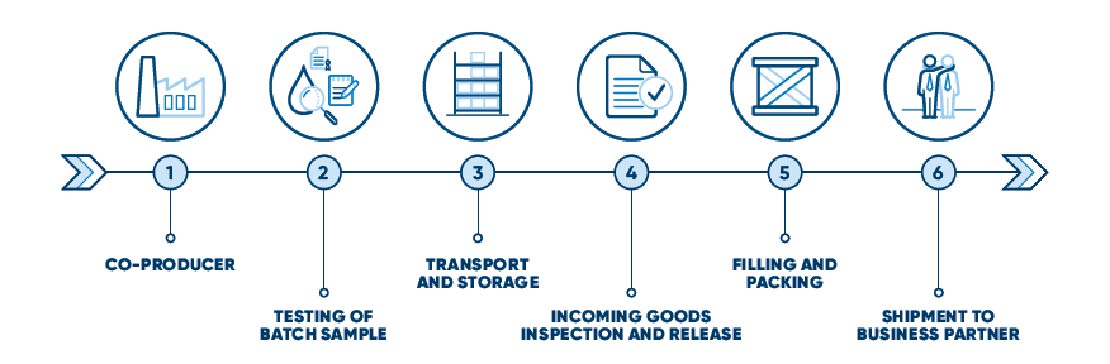
Our Solution - our Quality Process
Progress through Chemistry
Co-producer:
qualification of co-producer for sugar (derived from sugar beets), establishment of a purification step for co-producer to ensure high quality of sucrose.
Testing of batch sample:
testing of representative sample from produced sugar campaign to check EP, BP, NF, JP, ChP criteria plus microbiological parameters.
Transport and storage:
transport with own logistics from German sugar plant to qualified CG warehouse.
Incoming goods inspection and release:
sampling for microbiological limit testing (endotoxins, TAMC/TYMC) in CG`s own lab and partner labs, full testing according to pharmacopoeia (EP, BP, NF, JP, ChP).
Filling and packing:
- repacking of low endotoxin sucrose (filling line in a controlled environment at CG)
- storage in CG warehouse (wide range of packaging e.g. 1 kg, 5 kg, 10 kg, 12kg, 20 kg, 25 kg).
SUCROSE
Sucrose plays a vital role in the biopharmaceutical industry due to its versatile properties. It is widely used to stabilize proteins in formulations, protect cells and products during cryopreservation, and maintain product integrity during lyophilisation (freeze drying).
Sucrose also helps regulate osmotic pressure in cell culture media, stabilizes vaccine formulations, and serves as a bulking agent and taste masker in oral dosage forms. Additionally, it is used in parenteral formulations to adjust tonicity and ensure compatibility with physiological conditions.

Our Experts For you
Our Experts - Your Partners
Overview of key contacts - supported by many more specialists working for our partners and Clients behind the scenes.
Dr. Frank Velte
Head of CG Pharma & Biotech
+49 (0)511 87803-106
+49 (0)152 3466 7631
frank.velte@cg-chemikalien.de

Marco Regel
Head of Sales & Business Development
+49 (0)511 / 87803-146
+49(0)511 / 2516696
marco.regel@cg-chemikalien.de

Thomas Metzger
Sales & Business Development Manager
+49 (0)511 87803-258
+49 (0)79 483 72 81
thomas.metzger@cg-chemikalien.de

